The event that I am talking about is the Subway Sarin Incident. On March 20, 1995, domestic terrorists released gaseous sarin on several subway trains in the Tokyo Metro. Sarin is a nerve agent, a chemical that affects the nervous system. As a result, this terrorist attack killed 12 people and injured over 5,000 people. The victims of the attack experienced periods of temporary blindness. Many of them simply laid on the ground, unable to breathe. This is due to the fact that sarin attacks the central nervous system, paralyzing the lungs and thus causing difficulties throughout the respiratory system.
 |
A transit worker squats beside the body of a man after the sarin gas attack in the Tokyo subway.
|
Before we get into the nitty-gritty of Sarin's structural importance and its properties, we should first talk about nerve agents in general. Nerve agents can also be called neurotoxins, so don't be alarmed if I ever interchange the two words. Also, we should review the important parts of the nervous system to understand where and how sarin works. Now, what exactly is a nerve agent? Well, we know that it affects the nervous system. More specifically, a nerve agent is a phosphate-containing organic chemical, also known as an organophosphate. Nerve agents work by blocking the enzymes of neurotransmitters, found in the synaptic clefts between two neurons. Woah what did he just say?! Forgive me, reader, and Dr. Crane too if you're reading this, for I am about to talk about, dare I say it, biology. I promise I 'll try to be as brief as possible.
Now, in the nervous system there are nerve cells known as neurons. You can see a picture of a neuron down below. Neurons are essentially the building blocks of the nervous system. Neurons, in my opinion, are the coolest types of cells, as their structure is highly unique to that of other cells found in the body. Neurons are extremely important, as they are the only cells that can process and transmit information. They can send information in the form of electrical or chemical messages. The chemical messages are known as neurotransmitters, and these are what we're going to focus on when we later talk about sarin. Now, neurons are made up of three basic parts: cell bodies (soma), dendrites, and axons. There are billions of neurons throughout the human body, and remember, these neurons need to send information from one neuron to the next. They communicate when the axon of one neuron passes along information to the dendrites of another. Now, neurons in the body do not touch each other when they transmit information. There is a gap between each neuron called the synapse. Information must be transmitted across the synapse. In most cases, neurotransmitters are sent across synapses. When neurotransmitters leave one neuron, they must bind to the post-synaptic receptors located on the dendrites of the next neuron. After binding to their receptors, neurotransmitters are reabsorbed and reused by the neuron. Neurotransmitter-specific enzymes such as acetylcholinesterase (AChE) help aid this re-uptake process of neurotransmitters. I have just described to you in a nutshell the basis of neuronal messaging throughout the body's nervous system.
| Illustration of a neurons and neuronal messaging. |
 |
| Neurotransmitters traveling across a synapse. |
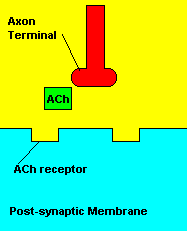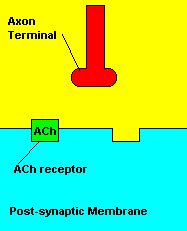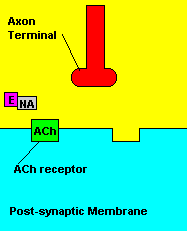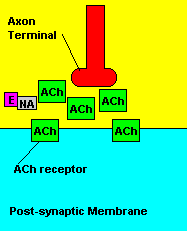
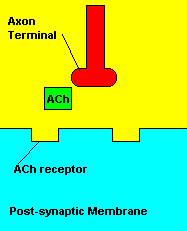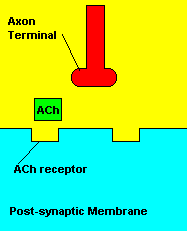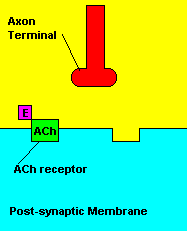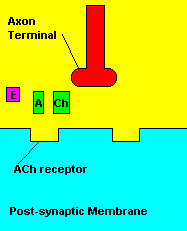
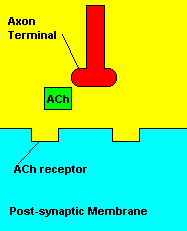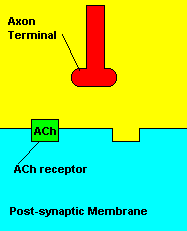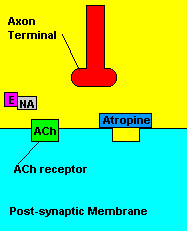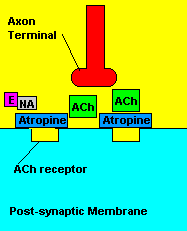
Normal transmission of acetylchline. Tranmission with Nerve Agent interference.
Sarin deals with the neurotransmitter known as acetylcholine. Acetylcholine is associated with memory, muscle contractions, and learning. A lack of this neurotransmitter, along with others, can lead to many neurodegenerative diseases such as Alzheimer's Disease or Dementia with Lewy Bodies. When acetylcholine is released from an axon terminal, it moves across a synapse to bind to its receptor on the other side of the synapse (on the post-synaptic membrane). In other parts of the body, acetylcholine is located in neuromuscular junctions, which are similar to synapses. Here, acetylcholine controls muscular contraction. As mentioned before, the action of acetylcholine is stopped by the enzyme acetylcholinerase (AChE). Sarin binds to AChE molecules and blocks the action of AChE. Again this means that there is no way to control the action of acetylcholine. Consequences of uncontrolled acetylcholine build-up include twitching, paralysis, respiratory failure, reduced vision, sweating, vomiting, headaches, comas, slurred speech, and unsteady muscular contractions, just to name a few.
 |
| Neurotransmitter acetylcholine |
 |
| Enzyme acetylcholinesterase (AChE) |
Now that we have a fairly solid understanding of how sarin works with enzymes and neurotransmitters, let's take it down one more level. Let's look at all of these phenomena on a molecular level. First, what exactly is sarin, in terms of atoms. Sarin's molecular formula is C4H10FO2P. Sarin is a chiral molecule, meaning that it can mirror itself. Another example of chirality would be your two hands. They are mirror images of one another. When you hold up your right hand in the mirror, it looks like your left hand. In chemistry, chiral molecules are typically ones where there are 4 groups attached to a center carbon atom. In a sarin molecule, there are four things attached to a central tetrahedral phosphorus atom. Sarin was first synthesized in 1938 during WWII. It is prepared from methylphsphonyl difluoride and a mixture of isopropyl alcohol. In other words:
CH3P(O)F2 +
(CH3)2CHOH → [(CH3)2CHO]CH3P(O)F + HF
The boiling point of sarin is around 158°C (316°F) and its melting point is -56°C (-69°F). Sarin is also miscible in water. Now the reason why sarin binds to acetylcholinesterase (AChE) has to do with acetylcholinesterase's structure. In its natural state, the enzyme is a monomer and has a molar mass around 60,000 g/mol. The enzyme has alpha/beta-linked proteins, as it contains 537 amino acids. The optimum pH for AChE is 7.0. Now what binds sarin to AChE? Well if look bag to the above picture of acetylcholine, the neurotransmitter appears to be positively charged. Therefore, acetylcholine has a high electron affinity for AChE. Likewise, sarin is the same. In addition, if we look at the below picture of sarin, we see lots of oxygen atoms and a fluorine atom at the end of the center phosphate atom. Given that AChe has hundreds of oxygen and hydrogen atoms, there is no doubt that there is immense hydrogen bonding between sarin and AChE. If we think about it, there are all these things in the synapse: acetylcholine, AChE, and sarin. AChE has to choose between sarin and acetylcholine, and obviously it chooses sarin, or sarin chooses AChE. Therefore, we can conclude that the intermolecular forces between sarin and AChE are much stronger than the ones between AChE and acetylcholine. Also, sarin has the end fluorine atom, which is the most electronegative atom. Again, this would contribute to its high electron affinity.
 |
| 3-D molecule of sarin |
2-D structure of sarin
Fortunately, there is a cure for sarin poisoning. A drug known as atropine has been used as an antidote. Atropine works by blocking one type of acetylcholine receptor, therefore rendering useless any excess acetylcholine that's floating around in the synapse.
 |
| Presence of atropine at the synapse. |
Nerve agents such as sarin are extremely dangerous. They
can kill people within minutes of exposure. Militaries from
many countries have used sarin. Germany during WWII, Iraq during the
Iraq-Iranian War, and so on. There is a paradox that states: "In order to
preserve peace, we must prepare for war." In order to sustain order in
this world, or in any world for that matter, be it the physical world, the
chemical world, the molecular world, we must find balance and hold on to it. As
humans, we've reached great heights. But do you think we've climbed too far this time? How much longer will it be until the next worst thing is developed? Similar to my previous post on Agent Orange, this post again highlights the theme that while science can bring so much good into this world, it can equally bring as much bad. Thank you for reading, and as always, have a nice day.
By: Max Lauring