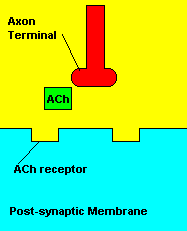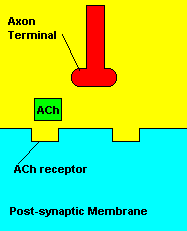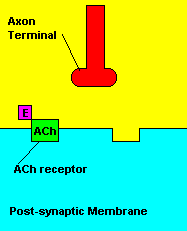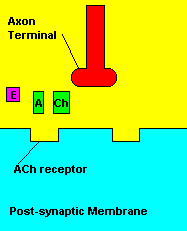
In the words of Austrian psychologist Sigmund Freud: "If it is admitted that art and science have the power to do good,
then it must also be admitted that they have the power to do harm."
Personally, I am not the most enthusiastic advocate of Freud's work. I disagree with
many of his theories pertaining to sex, the unconscious mind, and
hysteria. However, I believe there is hardly anything truer than his statement
- science, especially chemistry, has the potential to bring both good and bad into this world - order and chaos, construction
and destruction - and it is this potential that worries me at times.
My friends, I am about to take you through a brief history of the chemical
warfare that occurred during the Vietnam War (1960 - 1975). More
specifically, I am going to talk about the herbicide and defoliant known as
Agent Orange. What you are about to read highlights both the beauty and the
atrocity - the iniquity of Agent Orange and its everlasting mark on history.


Enough with the ominous and pessimistic introduction, and
onto the chemistry! Now, what exactly is Agent Orange? Agent Orange is the code
name for a powerful herbicide and defoliant developed primarily for military
usage. The military first used Agent Orange during the Vietnam War, mainly from
1961-1971. The herbicide is called "Agent Orange" because during the
war, it was shipped in orange-striped barrels. As you can probably guess, Agent
Orange was used to kill plants and crops on the ground, in addition to
defoliating the leaves off of plants and forest trees. Basically, it killed a
lot of plants and trees. It is estimated that nearly 20 million gallons of
Agent Orange were sprayed over various parts of Vietnam.
 |
| U.S. military planes spraying Agent Orange over miles of Vietnam's forests. |
Now, what is so bad about
Agent Orange? What makes it so harmful? We must first talk about the chemical
makeup of Agent Orange. Agent Orange is a 50:50 mixture of two chemicals known
as 2,4-D (2,4-dichlorophenoxyacetic acid or C8H6Cl2O3) and
2,4,5-T (2,4,5-trichlorophenoxyacetic acid or C8H5Cl3O3).
These two compounds can act like plant hormones. In case you forgot what a
hormone was, a hormone is essentially like a chemical messenger
that transports signals from one cell to another. Hormones can affect
the structure of organisms, such as the rigidity of cell walls in plants. By
acting like false plant hormones, 2,4-D and 2,4,5-T destroy plants by
interfering with their normal metabolism. When exposed to these substances,
plants can experience sudden, uncontrolled growth, kind of like a plant cancer.
Plants can't receive enough nutrients when growing uncontrollably, and
eventually shed their leaves and die.
 |
| 2,4-D |
 |
| 2,4,5-T |
Agent Orange not only harms
plants, but humans as well. This is due to the fact that the synthesis of Agent
Orange produces dioxins as a byproduct. Dioxins are a group of chlorinated organic
chemicals with similar structures. Some dioxins are extremely toxic while
others aren't. Why is this? Well, the potential harm a dioxin can induce
depends on the number and the position of the Cl atoms attached. The
dioxin that is the synthesis's byproduct is known as
2,3,7,8-tetrachlorodibenzo-p-dioxin, or TCDD. Unfortunately, TCDD has
been described by some scientists as "perhaps the most toxic
molecule evry synthesized by man." Since dioxins are carcinogenic to
humans, it is important to note how dioxins enter the human
body. Dioxins such as TCDD are emitted into the atmosphere and land in
water or on ground. In water, dioxins bind strongly to small particles and
other organic compounds. These molecules are inhaled by microorganisms, and
eventually the dioxins will find their way up the food chain until we eat an
organism with dioxin in it. Dioxins that land on the ground strongly bond with
the organic compounds in the soil, and therefore any present
groundwater isn't contaminated. Humans are also exposed to dioxins by air,
especially when they come into contact with dioxin-containing herbicides.
Dioxins are almost insoluble in water and have a high affinity for lipids
(fat), such as the fat stored in the human body. Lastly, dioxins are highly
stable due to their near perfect symmetry. Another group wrote about the symmetry of
dioxins and Agent Orange on its blog.


Now let's analyze the
structure and synthesis reactions of Agent Orange and its compounds. This part
will be more conceptual. For me, seeing pictures sometimes helps me more when
it comes to understanding certain material. It's helpful to see which atoms
move where. Also, there's that saying that a picture is worth a thousand
words...so I guess I could just have two pictures instead of this blog
post...anyway, as I mentioned before, Agent Orange is just a one-to-one mixture
of 2,4,5-T and 2,4-D. Dioxin is the unintentional byproduct of 2,4,5-T
synthesis. The picture below shows the step-by-step synthesis of 2,4,5-T. Note
that a key step in the formation of 2,4,5-T is the synthesis of
2,4,5-trichlorophenol.
 |
Synthesis of 2,4,5-trichlorophenol, which is
important in the formation of 2,4,5-T. |

On the left is the synthesis of 2,4,5-T, a defoliant and herbicide used to Agent Orange.
The picture on the left shows each step of the production of the byproduct dioxin or 2,3,7,8-T. Notice how the Cl atoms are attached to the molecule at the 2nd,3rd,7th, and 8th Carbon atoms in each ring. This is where it gets its name from.
The New York Times
published an article titled "Remedying the Effect of Agent Orange" in
August 2012. The article talked about the horrors of Agent Orange's effect. The
effects of Agent Orange are still seen today. Mothers in Vietnam still have
birth defects. Their children are mutated. Dioxin-induced cancer is still
present. The NY Times article stated that if we really want to help the victims
of the war, :we should begin by financing a more widespread cleanup and by
providing serious support to the families struggling with the birth defects
that are one of the legacies of our invasion of their country."
 |
Vietnamese army captain who was
exposed to Agent Orange during the
Vietnam War. |
 |
| Before dioxin poisoning. After dioxin poisoning. |
I am sorry if you finished reading this post with a
feeling of sadness or disgust. Unfortunately, this is life, and the
reality of it can sometimes be overwhelming. I hope you were able to learn
about the chemistry of Agent Orange and its mark on history. Unfortunately, Mr.
Freud was right: if science has the power to help others, it has the power to
do harm others. Feel free to comment about anything, and as always, have a nice
day.
By: Max Lauring